![PDF] Heat capacity and glass transition in P2O5-H2O solutions: support for Mishima's conjecture on solvent water at low temperature. | Semantic Scholar PDF] Heat capacity and glass transition in P2O5-H2O solutions: support for Mishima's conjecture on solvent water at low temperature. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/e9e458615e33c40d94b80a3189efa26d438c8a39/4-Figure2-1.png)
PDF] Heat capacity and glass transition in P2O5-H2O solutions: support for Mishima's conjecture on solvent water at low temperature. | Semantic Scholar

One mole of P2O5 undergoes hydrolysis as P2O5 + H2O⟶H3PO4 The normality of the phosphoric acid formed is (The volume of solution is 1 L)

One mole of P2O5 undergoes hydrolysis as P2O5 + H2O⟶H3PO4 The normality of the phosphoric acid formed is (The volume of solution is 1 L)

PDF) Phase Equilibria in the Yb2O3–P2O5–H2O System: Synthesis and Characterization of Ytterbium Phosphates

PDF) Heat capacity and glass transition in P2O5–H2O solutions: support for Mishima's conjecture on solvent water at low temperature | Federico Nores Pondal - Academia.edu
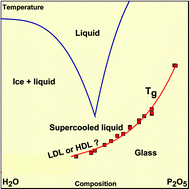
Heat capacity and glass transition in P2O5–H2O solutions: support for Mishima's conjecture on solvent water at low temperature - Physical Chemistry Chemical Physics (RSC Publishing)

CCLXXXI.—The systems B2O3–SO3–H2O and B2O3–P2O5–H2O - Journal of the Chemical Society (Resumed) (RSC Publishing)
![PDF] Heat capacity and glass transition in P2O5-H2O solutions: support for Mishima's conjecture on solvent water at low temperature. | Semantic Scholar PDF] Heat capacity and glass transition in P2O5-H2O solutions: support for Mishima's conjecture on solvent water at low temperature. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/e9e458615e33c40d94b80a3189efa26d438c8a39/4-Figure3-1.png)
PDF] Heat capacity and glass transition in P2O5-H2O solutions: support for Mishima's conjecture on solvent water at low temperature. | Semantic Scholar

Thermodynamic equilibrium in the system H2O+P2O5+CaCO3 at 25 and 70 °C: Application for synthesis of calcium phosphate products based on calcium carbonate decomposition - ScienceDirect
![PDF] Heat capacity and glass transition in P2O5-H2O solutions: support for Mishima's conjecture on solvent water at low temperature. | Semantic Scholar PDF] Heat capacity and glass transition in P2O5-H2O solutions: support for Mishima's conjecture on solvent water at low temperature. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/e9e458615e33c40d94b80a3189efa26d438c8a39/5-Table2-1.png)
PDF] Heat capacity and glass transition in P2O5-H2O solutions: support for Mishima's conjecture on solvent water at low temperature. | Semantic Scholar

The system Al2O3-P2O5-H2O at temperatures below 200 °C: Experimental data on the stability of variscite and metavariscite AlPO4·2H2O | Semantic Scholar

How to balance P2O5+H2O=H3PO4|Chemical equation P2O5+H2O=H3PO4|P2O5+H2O=H3PO4 balanced|P2O5+H2O= - YouTube

Thermodynamic equilibrium in the system H2O+P2O5+CaCO3 at 25 and 70 °C: Application for synthesis of calcium phosphate products based on calcium carbonate decomposition - ScienceDirect

How to balance P2O5+H2O=H3PO4|Chemical equation P2O5+H2O=H3PO4|P2O5+H2O=H3PO4 balanced|P2O5+H2O= - YouTube










